

Thalloo promotes the availability of in-vitro diagnostic (IVD) equipment in countries with high incidences of cancers and other conditions, focusing on Eastern Europe and the Baltic States.
Its mission is to innovate and enhance laboratory and point-of-care services to improve patient outcomes in both public and private healthcare sectors. Leveraging a robust network of clinical and medical expertise, industry specialists, and regulated professionals, Thalloo ensures the integrity and excellence of their international operations.
Through extensive manufacturer relationships and industry expertise, Thalloo supports access to cutting-edge diagnostics and testing solutions, maintaining high standards of probity and professionalism to meet the evolving needs of the global healthcare community.
Thalloo's mission is to innovate and promote
cutting-edge technologies that enhance the capabilities of laboratories and
point-of-care services, ultimately improving patient outcomes in both public
and private healthcare sectors.
We leverage a robust network of clinical and
medical expertise, industry sector specialists, and regulated professionals to
ensure the integrity and excellence of our international sales and financial
operations. Our commitment to maintaining high standards of probity and
professionalism drives us to deliver superior solutions that meet the evolving
needs of the global healthcare community.
By focusing on these three key areas, Thalloo effectively drives innovation and excellence in the global healthcare sector, fulfilling our mission to enhance patient outcomes through advanced medical technologies.

We actively invest in new and emerging companies that align with our mission of delivering advancing healthcare technologies. By providing loans and venture capital, we support their growth and the development of innovative solutions that have the potential to make a significant impact on patient care and healthcare delivery.

Thalloo forms strategic joint operations with entrepreneurs and local healthcare providers in our target countries. These partnerships allow us to extend our reach and deliver localised healthcare solutions that meet the specific needs of each community. Through these collaborations, we ensure that our advanced technologies and services are accessible to a broader audience.

We are committed to supporting clinics and hospitals by introducing new technologies and donating essential training and equipment. This sponsorship enables healthcare providers to adopt and utilise cutting-edge diagnostic and treatment tools, ultimately improving the quality of care they can offer to their patients.
We focus on working within Eastern Europe and the Baltic States. Our interest in supporting this region developed from the significant impact that the Chernoybl disaster of 1986 had on health in the region.
Our interest has since expanded to improving healthcare in the region by developing access to a wide range of diagnostics technologies that focus from cancer related testing through to other testing requirements, including most recently COVID-19.
Our attention remains on providing support to the healthcare requirements of Ukraine during the challenges and pressures that its healthcare system finds itself in under difficult circumstances.
Thalloo affirms its commitment to compliance with international sanctions and cross-border trade regulations. We have no operations, partnerships, or business engagements in Russia. Thalloo does not collaborate with manufacturers based in Russia, and our end-user partnerships do not serve customers within the Russian market. This stance reflects our support for Ukraine and adherence to global regulatory standards.
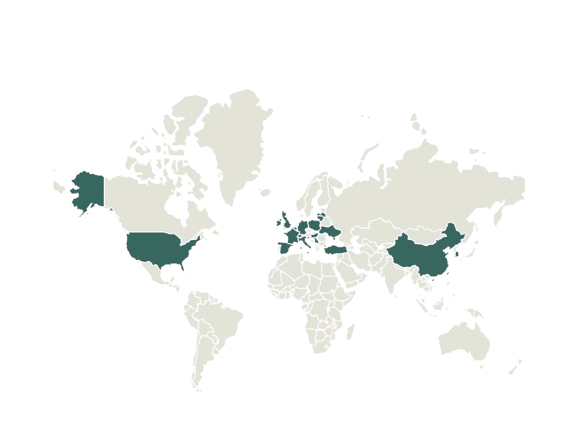
We are working with partners in over 20 countries to bring access to clinical diagnostics solutions into public and private healthcare laboratories; including from Belgium, China, Finland, France, Germany, Greece, Hungary, Ireland, Italy, Latvia, Lithuania, Poland, Slovenia, South Korea, Spain, Switzerland, Taiwan, Turkey, United Arab Emirates, United Kingdom and the United States.
Countries affected
by the Chernobyl nuclear disaster in 1986 continue to face exceptional healthcare needs due to the high incidence
of cancers and other conditions resulting from the radioactive fallout. The disaster
released large amounts of radioactive isotopes, leading to significant health
consequences, particularly among those
exposed at a young age.
Thyroid
cancer is the most significant health impact observed, with about 4,000 cases
primarily among those exposed as children or adolescents. These regions
continue to see higher rates of leukemia and other cancers.
The
healthcare systems in these areas must also address congenital malformations
and other long-term health effects potentially linked to radiation exposure.
The
long-term health monitoring of these populations is crucial as these risks
persist decades after the initial exposure. Consequently, clinical and medical diagnostics technologies play a critical role in monitoring and diagnosing the health implications for the population of the region over the long-term.
Implementing
advanced clinical diagnostics, up-to-date equipment, and sophisticated
diagnostic testing can significantly benefit healthcare systems in countries
affected by the Chernobyl disaster.
These
regions continue to grapple with high incidences of thyroid cancer and other
health conditions due to radiation exposure. Enhanced diagnostic tools, such as
high-throughput sequencing and advanced imaging technologies, allow for early
detection and precise characterization of cancers, leading to better treatment
outcomes and personalized care.
For instance,
next-generation sequencing has been used to study genetic mutations in thyroid
cancers caused by radiation from Chernobyl, helping to understand the specific
genomic alterations involved and guiding targeted therapies. Additionally, comprehensive diagnostic
equipment ensures accurate monitoring and management of radiation-induced
conditions, thereby improving patient prognosis and quality of life.
These advancements
not only aid in clinical management but also enhance epidemiological
surveillance, providing critical data for ongoing public health efforts and
research into the long-term effects of radiation exposure.
At Thalloo, we remain steadfast in our commitment to supply essential medical diagnostics and healthcare equipment to hospitals and laboratories across Eastern Europe, including Ukraine. We believe it is our duty to support patients and healthcare providers who rely on our products for critical medical services. Despite the significant challenges posed by international sanctions, we continue to operate with integrity and dedication to ensure that healthcare delivery is not compromised during these difficult times.
Thalloo can confirm that it is not involved in Russia as a region of interest – to be clear, Thalloo has no partners in Russia, does not work with manufacturers based in Russia; and our end user-facing partnerships do not have customers based in Russia.
Companies working in Eastern Europe and the Baltic States face significant
challenges while operating under international restrictions and sanctions
imposed due to the Ukraine-Russia conflict. The sanctions, aimed at limiting
Russia's economic capabilities, have resulted in complex regulatory
environments that businesses must navigate. These include restrictions on
exports and imports of a wide range of goods, especially those with dual-use
capabilities and advanced technologies.
Operating
under international sanctions requires stringent compliance with a complex
array of regulations. The UK and EU have implemented extensive export controls
and restrictions on goods that could potentially be used in military
applications, including certain medical technologies.
Sanctions
have disrupted traditional supply chains, with the additional administration of ensuring compliance with sanctions contributing to delays. Ensuring that medical supplies reach their destinations
on time requires meticulous planning and coordination.
Compliance with sanctions involves continuous monitoring and adaptation to evolving regulations. This includes maintaining up-to-date knowledge of sanctioned entities and goods, which can be resource-intensive. The introduction of new clauses prohibiting the re-exportation of certain goods adds another layer of complexity to operating in this region.
Thalloo has regular dialogue with its partners to ensure that they have robust processes in place to work within International regulations and the related restrictions. This includes monitoring updates to UK, EU and US lists; working closely with local customs agents to ensure any cross-border trade is within guidelines; and where required, working with Government agencies to clarify or seek approval where required.
Access to in-market partners that provide local installation, and regular servicing of equipment provides opportunities to ensure that equipment remains for its intended use.
In-vitro diagnostics (IVD) are critical tools in modern healthcare, enabling
the diagnosis and monitoring of diseases by analyzing samples such as blood,
urine, or tissue outside the human body. IVDs play a pivotal role in detecting
a wide array of health conditions, including cancers, infectious diseases, and
genetic disorders.
IVD has made a major contribution to the diagnosis and management of treatments for cancers.
Our interest started two decades ago in countries with the World’s highest incidence of gastric, breast and infantile cancers after the nuclear disaster at Chernobyl – Eastern Europe and the Baltic States.
IVD has developed for treatment of a much wider range of conditions and our interest is being extended throughout Europe and into Asia.
Recent technological advancements have significantly
enhanced the accuracy, speed, and accessibility of these diagnostic tools,
making them indispensable in both clinical and point-of-care settings.
The field of IVD has seen remarkable advancements in recent years. The
integration of molecular diagnostics, such as Polymerase Chain Reaction (PCR)
and Next-Generation Sequencing (NGS), has revolutionized disease detection and
monitoring. These technologies allow for the precise identification of
pathogens, genetic mutations, and biomarkers, facilitating early diagnosis and
personalized treatment plans.
For example, the development of CRISPR-based diagnostics has shown promise
in providing rapid and highly specific detection of genetic material from
pathogens, including viruses like SARS-CoV-2. Additionally, the use of
digital PCR (dPCR) has improved the quantification of low-abundance targets,
making it a powerful tool for cancer detection and monitoring.
Thalloo's mission to innovate and promote cutting-edge IVD technologies aligns with the evolving needs of the global healthcare community. By leveraging a robust network of clinical and medical expertise, industry specialists, and regulated professionals, Thalloo ensures the integrity and excellence of its international operations.
The advancements in IVD technology not only improve diagnostic accuracy and speed but also contribute significantly to patient outcomes in both public and private healthcare sectors. Through strategic investments, partnerships, and sponsorships, Thalloo continues to drive innovation and excellence in healthcare diagnostics.
IVD technologies are utilized across various medical disciplines, each application enhancing patient care through early and accurate diagnosis.
The COVID-19 pandemic (novel coronavirus SARS-CoV-2) emerged in late 2019 and rapidly spread worldwide, leading to significant global health, economic, and social impacts.
The pandemic resulted in millions of infections and deaths, overwhelming healthcare systems, and prompting unprecedented public health measures.
It has also accelerated the development and deployment of diagnostic technologies and treatments, highlighting the importance of global cooperation in addressing public health crises.
Thalloo worked with healthcare services in 3 countries to help deliver technologies to assist in their response to COVID-19, working with established and new partners to ensure timely delivery of equipment, tests and PPE.
The COVID-19 pandemic created unprecedented challenges for healthcare
systems across the EU and Ukraine. Advanced medical diagnostics technologies
played a crucial role in addressing these challenges, particularly in the realm
of medical testing. The rapid development and deployment of these technologies
significantly enhanced the capacity to detect and manage COVID-19 cases,
providing a critical backbone for the public health response.
Utilization of Existing Techniques
Existing diagnostic techniques, such as RT-PCR
(reverse transcription-polymerase chain reaction), became the gold standard for
COVID-19 testing. Laboratories across Europe scaled up their operations to meet
the surge in demand. The robustness and accuracy of RT-PCR tests made them
indispensable for identifying active infections, helping to control the spread
of the virus through timely isolation and treatment of patients.
The
pandemic also spurred innovation in diagnostic technologies. Rapid antigen
tests, which provide results in minutes, became widely used for mass screening
in various settings, including airports, schools, and workplaces. These tests
complemented RT-PCR by offering quicker, though slightly less sensitive,
results.
Additionally, advancements in molecular diagnostics, such as
CRISPR-based tests and next-generation sequencing, allowed for more detailed
understanding of the virus and its variants, aiding in effective public health
interventions.
Emerging
manufacturers from Asia, particularly China and South Korea, played a pivotal
role in meeting the global demand for diagnostic tests. Companies rapidly scaled up production and exported millions of test
kits to Europe. Their expertise in high-throughput testing and cost-effective
production was crucial in bridging the supply gaps, ensuring that European
healthcare systems had the necessary tools to combat the pandemic effectively.
The
influx of advanced diagnostics from both European and Asian manufacturers
ensured that healthcare providers could swiftly identify and isolate COVID-19
cases, thereby reducing transmission rates. The collaboration between
international and local manufacturers also highlighted the importance of global
trade and cooperation in addressing public health emergencies. This synergy not
only improved testing capacity but also accelerated the development of new
diagnostic tools, fostering innovation within the industry.
Ukraine's
response to the COVID-19 pandemic was marked by a robust public health
initiative and significant international support. These
efforts helped Ukraine manage the pandemic's impact effectively and improve
healthcare delivery during the crisis. Lithuania
demonstrated exemplary management of the COVID-19 pandemic through effective
containment measures, economic stimulus plans. Additionally,
Lithuania actively participated in regional cooperation, providing humanitarian
aid to other EU countries and its eastern neighbours.
Thalloo leveraged its extensive manufacturer relationships and industry expertise, combined with its network of clinical and medical professionals, to provide critical advice on COVID-19 testing solutions.
By collaborating with existing manufacturer partners and establishing new relationships with manufacturers across Asia, Thalloo developed robust supply-chain solutions that ensured the timely delivery of essential equipment, tests, and personal protective equipment (PPE).
As a result, Thalloo successfully assisted healthcare services in three countries, delivering innovative technologies to support their COVID-19 response efforts.

If you have interest in our mission then please contact us at: [email protected].
3rd Floor, Quay House, South Quay, Douglas,
IM1 5AR, Isle of Man
Company No. 011852V
Thalloo operates from the Isle of Man as a respected international base in the British Isles and a close neighbour to the United Kingdom and its support services. Consequently it shares a reputation for probity of arrangements in business. It also has a presence in many of those countries of its featured interest and operates in association with key international manufacturers.
The following references have been used for some of the content shown on the site.
This site was created with the Nicepage

